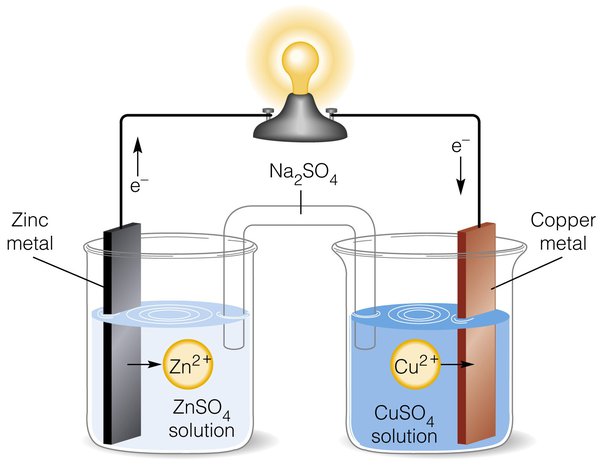
The positive electrode in certain electrochemical systems, like rechargeable batteries, needs continual replacement due to several factors:
- Repeated charge and discharge cycles cause chemical reactions that degrade the electrode material.
- Over time, the electrode’s ability to store and release energy diminishes, leading to reduced performance.
- Expansion and contraction during cycles can cause cracking and structural damage to the electrode.
- Unwanted side reactions can consume active materials and degrade the electrode.
- Some systems form protective layers that, paradoxically, become less conductive with time, reducing performance.
- Temperature, humidity, and contaminants can accelerate electrode degradation.
How do chemical reactions affect electrode degradation?
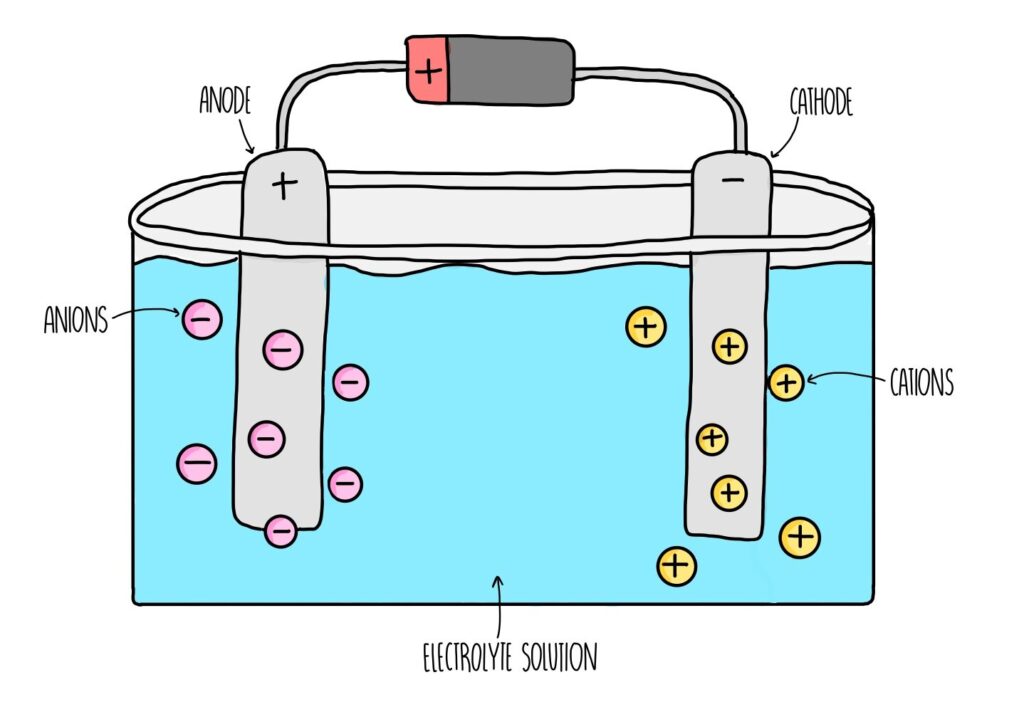
Electrochemical systems involve the flow of electrons and ions between the positive (cathode) and negative (anode) electrodes. During operation, chemical reactions occur at the electrodes.
In a rechargeable battery, for example, during discharge, the positive electrode typically undergoes a reduction reaction where it accepts electrons and ions, while during charging, it undergoes an oxidation reaction where it releases electrons and ions.
These chemical reactions, while essential for energy storage and release, can have detrimental effects over time.
The repeated charge and discharge cycles cause the electrode materials to expand and contract, leading to structural changes and micro-cracking. This can ultimately weaken the electrode’s integrity.
Capacity Fade
Definition and Significance of Capacity Fade: Capacity fade refers to the gradual reduction in the ability of the electrode to store and release electrical energy as the electrochemical system undergoes cycles of charge and discharge.
Additionally, it is a critical parameter in the lifespan and performance of batteries, affecting their energy storage capacity and runtimes.
Relationship to Electrode Performance: As capacity fades, the battery’s overall performance declines. It may hold less charge, discharge more rapidly, and require more frequent recharging.
Furthermore, capacity fade is a significant factor in determining when an electrode, particularly the positive electrode, needs replacement.
Mechanical Stress
Causes and Consequences of Mechanical Stress on Electrodes: Mechanical stress on electrodes results from the physical changes that occur during charge and discharge cycles.
As ions move in and out of the electrode material, it undergoes expansion and contraction. This mechanical strain can lead to electrode cracking and particle detachment.
Role in Electrode Degradation: Mechanical stress not only affects the physical integrity of the electrode but also influences the contact between active materials and current collectors.
Moreover, these mechanical disruptions can hinder the efficient flow of electrons, ions, and current, leading to reduced electrode performance and accelerated degradation.
Side Reactions
Explanation of Unwanted Side Reactions: In addition to the desired electrochemical reactions at the electrode, unwanted side reactions may occur.
Moreover, these side reactions can lead to the consumption of active electrode materials, reducing their availability for the primary electrochemical process.
Effects on Active Materials and Electrode Performance: Side reactions can result in the formation of undesirable compounds or irreversible chemical changes in the electrode material.
Moreover, these effects can cause capacity loss, decreased efficiency, and increased electrode degradation over time.
Passivation Layers
Formation and Purpose of Passivation Layers: Passivation layers, also known as solid-electrolyte interphases (SEI), can form on the electrode surface over time.
Moreover, these layers are initially intended to protect the electrode from further degradation and prevent unwanted reactions with the electrolyte.
Paradoxical Impact on Electrode Conductivity:
While SEI layers are protective, they can become thicker and less conductive as they age.
This paradoxical effect reduces the electrode’s electrical conductivity, limiting its ability to efficiently transfer electrons and ions during electrochemical processes.
Environmental Factors
Influence of Temperature, Humidity, and Contaminants: Environmental factors play a significant role in electrode degradation.
Elevated temperatures can accelerate chemical reactions and increase electrode wear, while high humidity levels can lead to corrosion.
Contaminants in the electrolyte or air can introduce impurities that promote unwanted reactions.
Acceleration of Electrode Degradation: Under unfavorable environmental conditions, electrode degradation can occur more rapidly, shortening the lifespan of the electrochemical system.
Moreover, managing and controlling these environmental factors are crucial for extending electrode life and overall system performance.
What minimizes electrode degradation in electrochemical systems?
Minimizing electrode degradation is critical for ensuring the longevity and efficiency of electrochemical systems, especially in applications like batteries and fuel cells.
Design Considerations
Strategies for Minimizing Electrode Degradation: Careful selection of electrode materials is crucial. Materials with high stability, low expansion/contraction, and resistance to chemical reactions can reduce degradation.
Designing the electrode structure to withstand mechanical stress is essential. This can involve using coatings, binders, or substrates that improve structural integrity.
Ensuring compatibility between the electrode material and the electrolyte is vital. Incompatible electrolytes can lead to side reactions and degradation.
Controlling the size and morphology of electrode particles can influence degradation. Smaller particles may offer better performance and mechanical stability.
Environmental Control
Importance of Maintaining Suitable Environmental Conditions: Controlling the environment where electrochemical systems operate is critical for minimizing degradation.
Maintaining a suitable temperature range helps regulate the kinetics of chemical reactions. Extremes in temperature can accelerate degradation or reduce performance.
High humidity can lead to corrosion and damage to the electrode materials, while excessively dry conditions can affect ionic conductivity.
Keeping the environment free from contaminants, such as dust or impurities in the electrolyte, prevents unwanted reactions and degradation.
Monitoring and Maintenance
Role of Monitoring Electrode Performance: Regularly monitoring the performance of electrodes is essential for early detection of degradation.
Tracking changes in capacity and voltage during charge and discharge cycles can indicate electrode health.
This technique measures changes in electrode impedance, which can reveal structural and chemical changes.
Subjecting electrodes to controlled cycling and analyzing performance over time can provide insights into degradation trends.
Approaches to Electrode Maintenance: Implementing maintenance schedules that include electrode inspections, cleaning, and replacement when necessary can extend the system’s life.
In some cases, electrodes can be regenerated by processes like electrodeposition, which can reverse certain types of degradation.
Continuously monitoring and controlling environmental conditions can help prevent degradation and maintain electrode performance.
Ongoing research into advanced electrode materials, coatings, and manufacturing processes can lead to improved electrode longevity and performance.
How can we recognize when to replace positive electrodes?
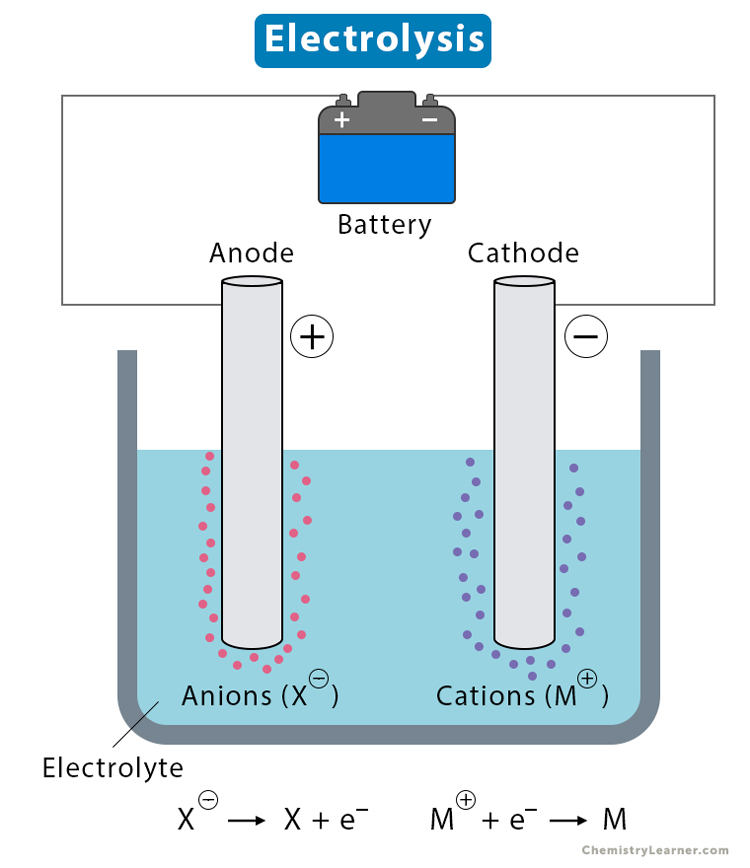
It’s essential to follow manufacturer guidelines and safety precautions when replacing positive electrodes, as the specific procedures may vary depending on the type of electrochemical system and its intended application.
Indications for Replacement
Recognizing When Replacement is Necessary: Several indicators can signal the need for positive electrode replacement in electrochemical systems:
- Capacity Loss: A noticeable reduction in the system’s capacity, meaning it can hold and deliver less energy, is a clear sign.
- Voltage Irregularities: If the voltage profile during charge and discharge cycles becomes unstable or exhibits abnormal behavior, it suggests electrode degradation.
- Increased Internal Resistance: Rising internal resistance, detected through impedance measurements, can hinder the flow of current and indicate electrode issues.
- Overheating: If the system overheats during operation, it may result from electrode degradation causing inefficient energy conversion.
- Reduced Efficiency: A decline in the overall efficiency of the system, where more energy is lost as heat, can point to electrode problems.
Procedures for Replacement
Steps Involved in Replacing Positive Electrodes: The replacement of positive electrodes can be a complex procedure, depending on the specific electrochemical system. Here’s a general outline of the steps involved:
- Shutdown and Safety: Safely power down the system and follow safety protocols to prevent accidents.
- Electrolyte Draining: Remove or discharge the electrolyte from the system to avoid chemical reactions during replacement.
- Electrode Removal: Carefully disassemble the system to access the positive electrode.
- Cleaning and Preparation: Clean any remaining electrolyte or debris and prepare the electrode compartment.
- Electrode Replacement: Introduce the new positive electrode into the system, ensuring proper alignment and connection.
- Reassembly: Reassemble the system, taking care to secure all components and connections.
- Electrolyte Refill: Add fresh electrolyte to the system, following manufacturer guidelines for the correct type and quantity.
- Testing and Calibration: Test the system’s performance, calibrate sensors, and monitor its behavior to ensure proper functioning.
FAQ’s
The anode in various electrochemical systems, like water heaters, can corrode over time due to its sacrificial role, protecting the cathode (metal surface). Replacing it is necessary to maintain the protection and prevent damage to the cathode.
Anodes should be replaced when they are significantly corroded, typically when they are about 50% consumed. Regular inspection is crucial to determine the right time for replacement.
If you don’t replace a corroded anode rod in a water heater, the tank itself may start corroding, leading to leaks and reduced efficiency, which can be costly to repair or replace.
In electrolysis, the anode corrodes as it donates electrons to the solution. Replacing it ensures the process continues efficiently and prevents contamination of the electrolyte.
No, in electrolysis, the anode is indeed the positive electrode where oxidation occurs, but in other electrical systems like batteries, the anode can be either positive or negative depending on the type of battery.
The anode must be at a positive potential to attract negatively charged ions or electrons in the electrochemical process, allowing oxidation reactions to occur at the anode, where electrons are lost. This is essential for many electrochemical processes, including corrosion protection and electrolysis.
Final Words
When it comes to electrochemical systems like batteries, replacing the positive electrode is crucial to maintain their performance and longevity. Knowing when it’s time for replacement is essential, and you can look for signs like reduced capacity, unstable voltage, or overheating.
Moreover, when it’s time to replace, follow the proper procedures carefully. This involves safely shutting down the system, removing old electrodes, cleaning, installing new ones, and testing everything to make sure it’s working well.
In addition, timely replacement brings many benefits, including restoring performance, extending the system’s life, ensuring safety, and saving money in the long run. So, keep an eye on your electrodes, and when they need it, don’t hesitate to give them a fresh start!