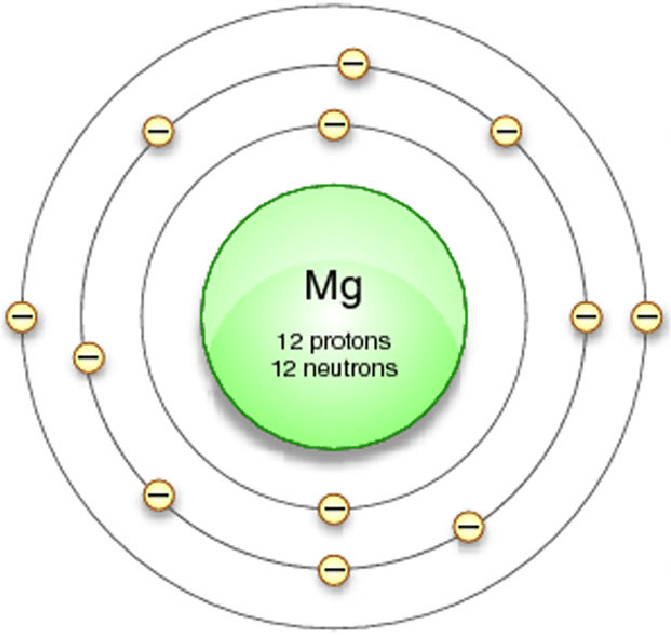
Magnesium, a chemical element represented by the symbol Mg on the periodic table, has an atomic number of 12. The atomic number of an element tells us the number of protons in its nucleus, and since atoms are electrically neutral, it also corresponds to the number of electrons in the atom.
So, how are these 12 electrons in magnesium distributed across its energy levels?
First Energy Level (K-shell): This is the innermost energy level and can hold a maximum of 2 electrons. In the case of magnesium, it has 2 electrons in the first energy level.
Second Energy Level (L-shell): The second energy level is further from the nucleus and can hold a maximum of 8 electrons. Magnesium accommodates 8 electrons in its second energy level.
Third Energy Level (M-shell): Even farther from the nucleus is the third energy level, which can also hold a maximum of 8 electrons. However, magnesium only contains 2 electrons in its third energy level.
How does atomic structure relate to an element’s properties?
The basic atomic structure is fundamental to understanding the properties of elements and their behavior in chemical reactions. At the core of this structure is the nucleus, a small, dense region located at the center of an atom. The nucleus contains two types of subatomic particles: protons and neutrons.
Surrounding the nucleus are electrons, which are much smaller in mass compared to protons and neutrons. Electrons are negatively charged and are found in regions called electron shells or energy levels.
Explanation of Protons, Neutrons, and Electrons
Protons: Protons are positively charged subatomic particles found within the nucleus of an atom.
Each element has a unique number of protons, known as its atomic number, which defines its identity.
The number of protons determines the element’s chemical properties and its position on the periodic table. In the case of magnesium, its atomic number is 12, meaning it has 12 protons in its nucleus.
Neutrons: Neutrons are electrically neutral subatomic particles also located in the nucleus.
The number of neutrons can vary for atoms of the same element, leading to the existence of isotopes.
In addition, neutrons help stabilize the nucleus by counteracting the electrostatic repulsion between protons due to their positive charges. The total number of protons and neutrons in the nucleus is referred to as the mass number.
Electrons: Electrons are negatively charged and occupy specific energy levels around the nucleus. These energy levels are often referred to as electron shells or orbitals. Electrons are organized into sublevels (s, p, d, f) within each energy level.
Furthermore, the distribution of electrons within these energy levels and sublevels is described by the electron configuration.
What is the significance of magnesium’s atomic number?
The atomic number is a fundamental property of an element, and it plays a critical role in defining the identity of that element. It is defined as the number of protons found in the nucleus of an atom of that element.
In other words, the atomic number is a unique whole number assigned to each element, and no two elements have the same atomic number.
Explanation of How Atomic Number Determines the Number of Protons in an Element
Protons and Atomic Number: Each element is composed of atoms, and these atoms have a specific number of protons in their nucleus.
The atomic number is equal to the number of protons in the nucleus of an atom of that element.
For example, if we look at hydrogen, which has an atomic number of 1, this means that a hydrogen atom contains one proton in its nucleus.
Unique Identity: Because the number of protons in an atom’s nucleus is unique to each element, the atomic number serves as the element’s “fingerprint.”
Additionally, it distinguishes one element from another and is the basis for organizing elements on the periodic table.
Mention of Magnesium’s Atomic Number
Magnesium has an atomic number of 12. This means that every atom of magnesium contains 12 protons in its nucleus. This characteristic atomic number is what defines magnesium as an element and distinguishes it from all other elements.
Furthermore, it is this number that allows us to identify magnesium on the periodic table and understand its chemical properties.
How is electron configuration determined in an atom?
Electron configuration is a fundamental concept in chemistry that describes the arrangement of electrons within an atom. It provides information about how electrons are distributed in various energy levels and sublevels around the nucleus of an atom.
The electron configuration notation helps us understand an element’s chemical behavior and its reactivity with other elements.
Distribution of Electrons in Energy Levels and Sublevels
Energy Levels (Principal Quantum Numbers): Electrons are organized into energy levels, labeled with principal quantum numbers (n = 1, 2, 3, …).
Energy levels are arranged in increasing order of energy, with the first level (n = 1) being closest to the nucleus and having the lowest energy.
As you move to higher energy levels, the electrons are found farther from the nucleus and have higher energy.
Sublevels (Angular Momentum Quantum Numbers): Each energy level contains one or more sublevels, often referred to as orbitals.
Sublevels are labeled using letters (s, p, d, f) and are associated with different shapes and orientations.
The s sublevel is spherical, the p sublevel has dumbbell-shaped orbitals, the d sublevel has complex shapes, and the f sublevel has even more complex shapes. The number of sublevels in an energy level is equal to the principal quantum number (n).
Detailed Electron Configuration of Magnesium
Now, let’s specifically look at the electron configuration of magnesium (atomic number 12):
First, determine the number of electrons in magnesium. Since its atomic number is 12, it has 12 electrons.
Electron distribution: The first energy level (n = 1) can hold a maximum of 2 electrons, so the first two electrons go into the 1s sublevel.
- The second energy level (n = 2) can hold a maximum of 8 electrons. After the 1s sublevel, the remaining 10 electrons are distributed as follows:
- 2 electrons in the 2s sublevel.
- 8 electrons in the 2p sublevel (2 electrons in each of the three 2p orbitals).
The electron configuration of magnesium is represented as follows:
1s² 2s² 2p⁶
However, this notation indicates that magnesium has 2 electrons in its 1s sublevel, 2 electrons in its 2s sublevel, and 6 electrons in its 2p sublevel.
What role do valence electrons play in chemical reactions?

Valence electrons are the electrons located in the outermost energy level or shell of an atom. They are the electrons that are involved in chemical reactions and interactions with other atoms.
In addition, the valence electrons determine an element’s chemical properties, reactivity, and its ability to form chemical bonds with other elements.
Explanation of the Role of Valence Electrons in Chemical Reactions
Reactivity: Valence electrons are responsible for an element’s reactivity. Elements with similar numbers of valence electrons often exhibit similar chemical behaviors and reactions.
Moreover, the number of valence electrons influences how readily an atom can gain, lose, or share electrons to achieve a stable electron configuration.
Chemical Bonding: Atoms tend to form chemical bonds to achieve a full outer electron shell, typically with 8 electrons (known as the octet rule), resembling the electron configuration of noble gases.
Elements with a few valence electrons are more likely to lose them and become positively charged ions (cations).
Elements with several valence electrons are more likely to gain electrons and become negatively charged ions (anions). Sharing of valence electrons leads to covalent bonds in molecules.
Impact on Chemical Properties:
The number and arrangement of valence electrons determine an element’s chemical properties, such as its ability to conduct electricity, react with other substances, and form compounds.
Identification of the Number of Valence Electrons in Magnesium
Magnesium (atomic number 12) has its valence electrons located in the outermost energy level, which is the second energy level (n = 2).
To determine the number of valence electrons in magnesium, you look at its position on the periodic table. Magnesium is in Group 2 (Group IIA) of the periodic table. Elements in this group have 2 valence electrons.
In addition, this is because the number of valence electrons corresponds to the group number for elements in the s-block of the periodic table.
So, magnesium has 2 valence electrons.
These two valence electrons in magnesium’s outermost energy level play a crucial role in its chemical reactivity, as magnesium readily loses these electrons to achieve a stable electron configuration.
Furthermore, this behavior makes magnesium a reactive metal and a common component of many chemical compounds and alloys.
FAQ’s
How many electrons does Mg2+ have?
Mg2+ has 10 electrons.
Why does magnesium have 2 electrons?
Magnesium, in its neutral state (Mg), has 12 electrons because its atomic number is 12. It loses 2 electrons to become the Mg2+ ion, which is more stable.
Does magnesium have 25 electrons?
No, magnesium has 12 electrons in its neutral state, as determined by its atomic number on the periodic table.
Why does Mg2+ have 10 electrons?
Mg2+ has 10 electrons because it loses 2 electrons from its neutral state (Mg) to achieve a stable, full outer electron shell.
Why does Mg2+ have a +2 charge?
Mg2+ has a +2 charge because it loses 2 electrons from its neutral state. This loss of electrons results in a positive charge on the ion.
How many electrons are in Mg2+ and why?
Mg2+ has 10 electrons because it starts with 12 electrons in its neutral state (Mg) and loses 2 electrons to achieve a stable electron configuration with a full outer shell.
Final Words
In conclusion, we’ve learned that understanding an element’s atomic structure is like knowing its own unique identity card. Elements have a special number called the atomic number, which tells us how many protons they have in their tiny central nucleus. This atomic number helps us find where an element belongs on the periodic table and explains its chemistry.
Moreover, we also looked at the electron configuration, which is like knowing how electrons sit around the nucleus. For magnesium, it’s 12 electrons distributed into different energy levels and sublevels, which influences how it behaves in chemical reactions.
Lastly, we explored valence electrons, which are like the outermost buddies of an element. Magnesium has 2 valence electrons, and these little pals are responsible for its reactivity and bonding with other elements.
So, understanding these concepts helps us unlock the secrets of the element magnesium and why it behaves the way it does in the world of chemistry.